Today there is no cure for Multiple Sclerosis (MS). FSD Pharma will attempt to change that for millions of suffering people. FSD’s proprietary compound Lucid-MS has shown promising results to reverse progressive MS, in several animal models
LUCID-MS
| Idea/Target | Lead | In vivo PoC | IND Enabling Studies | Phase 1 | Phase 2 | Phase 3 | Launch | |
|---|---|---|---|---|---|---|---|---|
| LUCID-MS |  Multiple sclerosis Multiple sclerosis |
Phase-1 in human trials completed | ||||||
LUCID-MS
Patented new chemical entity (NCE)
Lucid-MS is a patented neuroprotective compound that has demonstrated in preclinical models to prevent and reverse myelin degradation, a cause for Multiple Sclerosis, as well as other neurodegenerative diseases and conditions. Lucid-MS has shown excellent results in several animal models, as demonstrated in the video below.
LUCID-MS: Disease Modifying Enabling Remyelination
The subject in the video above is only one example taken from a large cohort of mice in experiments conducted in multiple labs across several years. The results of the preclinical research from these experiments has been published in various prestigous medical journals, including Journal of Medicinal Chemistry and Proceedings of the National Academy of Sciences (PNAS). Lucid-MS is a patented (patent #WO2017027967A1) New Chemical Entity (NCE) that has been studied extensively throughout more than 11 years of research and development. FSD Pharma owns exclusive worldwide rights to Lucid-MS.
A sampling of the novel qualities of Lucid-MS include:
Excellent efficacy in various preclinical animal models
Accelerates functional recovery of mouse models of Multiple Sclerosis, preserves myelin, and reduces zonal degradation
Does not suppress immune system (non-immunomodulatory)
Potential oral administration with easy dosing regimen
FSD Pharma has completed the dosing of healthy human volunteers for phase-1 clinical trials for Lucid-MS.
Today there is no cure for Multiple Sclerosis.
FSD Pharma (“HUGE”) is trying to change this for millions of people.

Multiple Sclerosis Overview
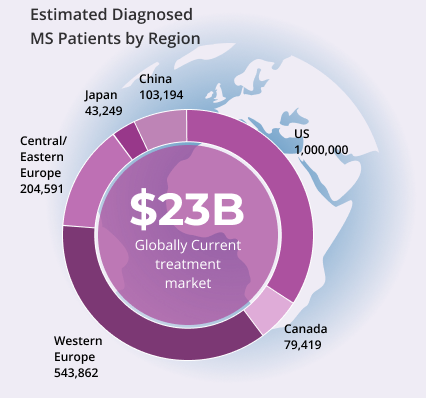
- A chronic inflammatory and degenerative disorder of the Central Nervous System Global prevalence of MS rose from 2.3 million in 2013 to 2.8 million in 2020
- MS is characterized by unpredictable symptoms (i.e. tingling sensations, vision problems, mobility issues) that are attributed to the patient’s immune system attacking different nerve fibers
- Damage to myelin (the sheaths protecting nerve fibers) can affect critical thinking and cognitive skills
- Global MS treatment market in 2022 estimated at US$23 billion
- MS can occur at any age, but the average age for diagnosis globally is 32 years 1.5% of the global MS population is under the age of 18 years
- Female to male diagnosis ratio is 2:1
- Prevalence in U.S. is 288 cases per 100,000 people; in Canada it is 250 cases per 100,000 people
Armed with a strong balance sheet, solid pipeline, and renowned clinical team, FSD intends to advance its lead drug candidates through clinical trials with the goal of providing therapies to the millions suffering from neurodegenerative and neuropsychiatric conditions.
Well positioned to advance current pipeline and aggressively pursue additional acquisitions that present compelling opportunities across the innovative biotech space focused on “Total Brain Health”
Renowned clinical team led by Dr. Lakshmi P. Kotra, who is the recipient of the Julia Levy Award, is a Senior Scientist at Krembil Brain Institute, as well as University Health Network (UHN), and Professor of Medicinal Chemistry at the University of Toronto. Dr. Kotra serves as CEO of FSD Pharma’s wholly owned subsidiary, Lucid Psycheceuticals
Investor Inquiries
Please send investor relations inquiries to the following email:

